You have no items in your shopping cart.
Why COA and MSDS Matter When Choosing a Culture Medium
Compliance and Documentation Considerations for PI and Lab Managers
When selecting a culture medium for routine research or plant transformation experiments, most discussions focus on formulation, performance, and reproducibility. However, for principal investigators and laboratory managers, regulatory documentation and traceability are equally important.
This article explains why Certificates of Analysis (COA) and Material Safety Data Sheets (MSDS) are essential when choosing a culture medium, and how proper documentation supports experimental reliability, laboratory compliance, and purchasing decisions.
What Is a COA and Why Does It Matter?
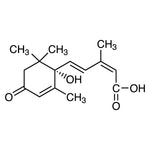
A Certificate of Analysis (COA) provides batch-specific quality information for a given product. For culture media used in Agrobacterium experiments, a COA typically documents:
- 📄 Lot number and traceability: Ensures experimental reproducibility across batches.
- 🧪 Quality control parameters: pH, appearance, and formulation consistency.
- 📊 Release criteria: Confirmation that the product meets predefined specifications.
Lack of COA documentation is a common issue encountered during audits or when troubleshooting unexpected Agrobacterium culture failures .
Why MSDS Is Essential for Laboratory Compliance
The Material Safety Data Sheet (MSDS) provides critical safety information required by institutional regulations and occupational safety guidelines. For laboratory managers, MSDS documentation supports:
- 🛡️ Chemical hazard identification: Safe handling and storage procedures.
- 🧯 Emergency response planning: Spill, exposure, and disposal guidance.
- 📋 Institutional compliance: Required for internal safety reviews and inspections.
Media products without accessible MSDS documentation may be restricted or rejected during procurement review, regardless of their reported experimental performance.
Documentation as a Purchasing Decision Factor
For procurement teams, COA and MSDS availability often determines whether a product can be approved for purchase. Compared with generic media, products such as YM Medium compared with LB medium offer added value by combining optimized formulation with transparent quality documentation.
This documentation-first approach also supports long-term consistency in applications such as Agrobacterium-mediated plant transformation , where batch variation can significantly impact results.
How YM Medium Supports Compliance and Research Confidence
YM Medium is supplied with complete COA and MSDS documentation, enabling laboratories to meet internal compliance requirements while maintaining high experimental standards. This documentation complements its role in improving Agrobacterium electroporation efficiency and overall culture stability.