You have no items in your shopping cart.
What Is a His-Tag?
A comprehensive guide to its principles, optimization, and applications in protein biochemistry.
A His-tag is a short stretch of 6–10 histidine residues added to a recombinant protein to enable affinity purification, detection, and biochemical assays. His-tags bind divalent metal ions such as nickel (Ni²⁺) or cobalt (Co²⁺), which makes it possible to selectively capture tagged proteins using metal-chelated agarose beads.
Why it matters: Because of their simplicity, robustness, and low cost, His-tags have become one of the most widely used affinity tags in protein biochemistry today.
Why use a His-tag?
Before recombinant DNA technology, proteins were commonly purified from animal tissues using labor-intensive methods based on size and charge. The development of genetic cloning transformed protein production by enabling recombinant expression in hosts such as Escherichia coli.
During plasmid design, affinity tags can be genetically fused to the protein of interest. These tags act as molecular “handles,” allowing rapid and selective purification. The His-tag is particularly popular due to its small size and strong interaction with metal ions.
💡 Analogy: Think of it like airport luggage. When many suitcases look similar, a unique luggage tag allows you to quickly identify and retrieve your own. A His-tag allows you to isolate your target protein from a complex mixture of host proteins.
His-tag interaction with nickel ions
The imidazole side chain of histidine residues coordinates nickel ions. When multiple histidines are placed in sequence, the His-tag binds strongly to nickel-chelated agarose beads, while most non-tagged proteins pass through the column.

Figure 1. Contaminating host proteins containing natural histidine residues can bind to nickel beads in the absence of imidazole.
How long should a His-tag be?
Most His-tags contain 6–10 histidine residues. Increasing tag length strengthens binding to nickel beads due to avidity—multiple histidines coordinate the metal ion simultaneously.

Figure 2. Longer His-tags act like having "more hands," providing a stronger grip and more reliable capture.
Optimizing Purification with Imidazole
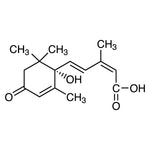
Imidazole competes with histidine for nickel binding. By strategically adjusting its concentration, you can achieve superior purity:
- ✔ Low concentrations (5–30 mM): Used in wash buffers to reduce non-specific binding.
- ✔ High concentrations (150–500 mM): Used to elute the target protein.

Figure 3. Imidazole prevents contaminating host proteins from binding to nickel agarose beads.
His-tags vs Other Affinity Tags
While alternatives like Strep-tag, FLAG-tag, GST, or MBP exist, His-tags remain the gold standard due to:
| Advantage | Benefit |
|---|---|
| Small Size | Minimal impact on protein structure/function. |
| Low Cost | Affordable and widely available reagents. |
| Versatility | Compatible with most biochemical assays. |

Figure 4. Tag removal via protease cleavage can restore enzyme activity if the tag interferes with the active site.
Broader Applications
Beyond purification, His-tags are foundational for:
- ELISA & Western Blotting
- Fluorescent Labeling
- SPR Immobilization
- Pull-down Assays
- FRET Studies
- Microscopy