You have no items in your shopping cart.
Revolutionary Stability: Diacylglycerol & Biofargo Gels Unlock New Emulsion Potential
Troubled by phase separation or water release in your whipped creams or emulsions at room temperature?
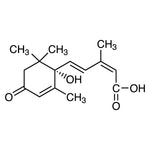
A groundbreaking study points to Diacylglycerol (DAG) as the key to long-term stability.
The Challenge: Room-Temperature Emulsion Instability
Compared to refrigerated aerated emulsions, room-temperature versions offer superior convenience for storage and distribution, eliminating the need for cold chain logistics. However, higher storage temperatures and environmental fluctuations often accelerate Brownian motion and fat globule collisions, leading to common instability issues like water separation, flocculation, and coalescence.
Previous studies hinted that using diacylglycerol (DAG) as a base oil could significantly enhance emulsion stability over time, yet the precise mechanism remained a mystery – until now.
Unveiling the Mechanism: A Multiscale Breakthrough
In their recent publication titled “Elucidating interaction mechanisms of protein and diacylglycerol at the interface on the static storage stability of partially crystalline emulsions through a multiscale approach”, the research team from the Oil Biorefinery and Nutrition Innovation Lab at Jinan University provided critical new insights into the interfacial behavior of DAG and proteins.
Utilizing a multiscale analytical approach—from macroscopic observations to molecular-level details—the study systematically investigated how proteins and DAG interact at the emulsion interface. Key findings revealed that PO-DAG (palm olein-derived DAG) specifically adsorbs casein through hydrogen bonding, effectively thickening the interfacial membrane of fat globules.
Further molecular data showed that DAG has a lower interaction energy barrier with casein, promoting a higher concentration of casein at DAG-rich interfaces. Through sophisticated interfacial rheology and Quartz Crystal Microbalance with Dissipation (QCM-D) analyses, the team explored micro-interactions, demonstrating that an optimal amount of DAG enhances membrane integrity. Conversely, excessive DAG can compete with proteins for interfacial sites, and its active hydroxyl groups may reduce the interfacial membrane's viscoelasticity.
These findings align closely with established electrophoretic characterization strategies, where selecting appropriate separation conditions—such as Native PAGE versus SDS-PAGE—is critical for preserving protein–lipid interaction states during analysis.

Visualizing the DAG-Casein interaction that enhances emulsion stability.
Accelerate Your Research with Biofargo
In this multi-layered, high-precision investigation, accurate analysis and verification of protein components were critical. To ensure high-resolution protein separation and reliable results, the research team relied on optimized electrophoresis workflows while avoiding common issues such as localized gel overheating and run instability, as discussed in our SDS-PAGE overheating mitigation guide.
Specifically, our Hepes-Tris Precast Gels (GSH2001-15T) played a vital role in electrophoresis experiments. Proper handling and storage of precast gels—including refrigeration rather than freezing—are essential for maintaining performance consistency, as detailed in our precast gel storage best practices.
Explore Hepes-Tris Precast Gels