You have no items in your shopping cart.
HSP90 Molecular Chaperone: Protein Folding, Stress Response, and Cancer
Exploring the central regulator of cellular proteostasis and its implications in modern disease therapy.
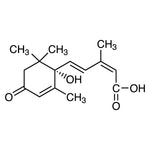
Heat shock protein 90 (HSP90) is a highly conserved molecular chaperone that plays a central role in protein folding, stabilization, and functional regulation. Abundantly expressed in eukaryotic cells, it is essential for maintaining cellular proteostasis under both physiological and stress conditions.
HSP90 regulates the maturation and stability of numerous client proteins involved in signal transduction, cell cycle control, and transcriptional regulation. Due to its involvement in multiple oncogenic and stress-response pathways, HSP90 has emerged as a critical regulator of cellular homeostasis.
I. Structural Organization & Characteristics
HSP90 functions as an ATP-dependent molecular chaperone that forms homodimeric complexes. Each monomer consists of three functional domains:
- • N-terminal domain: Contains ATP-binding pocket and drives conformational cycling.
- • Middle domain: Responsible for client protein interaction and ATP hydrolysis regulation.
- • C-terminal domain: Mediates dimerization and co-chaperone recruitment.
II. Protein Folding and Proteostasis
Proper protein folding is essential for enzymatic activity. HSP90 assists in the maturation of metastable proteins that require precise structural organization.
Key Client Proteins
- Hormone receptors (GR, ER)
- Protein kinases
- Transcription factors
- Cell cycle regulators
Proteostasis Mechanisms
- Promoting refolding
- Facilitating degradation
- Preventing toxic aggregation
- Quality control coordination
III. Cellular Stress Response Pathways
Exposure to heat shock, oxidative stress, or hypoxia induces protein denaturation. HSP90 expression is upregulated to mitigate proteotoxicity, working in coordination with:
HSP70 Family + Small HSPs + Co-chaperone Complexes
IV. Disease Implications
| Field | Key Pathological Drivers |
|---|---|
| Cancer Progression | PI3K–AKT signaling, RAS–RAF–MAPK, HER2 receptor, and Mutant p53 stabilization. |
| Neurodegeneration | Tau aggregation (Alzheimer’s), α-synuclein (Parkinson’s), and Polyglutamine misfolding (Huntington’s). |