You have no items in your shopping cart.
Product Description
This is an indirect-competitive ELISA kit for quantitative determination of kanamycin residues in plasmid DNA and related biologic samples (including cell and gene therapy products). Microplate wells are coated with kanamycin antigen which competes with kanamycin in the sample for a monoclonal anti-kanamycin antibody. An enzyme-tagged secondary antibody binds the primary antibody; addition of TMB chromogenic substrate yields a colorimetric signal at 450 nm that is inversely proportional to kanamycin concentration. Total kanamycin is determined from a standard curve (4-parameter logistic) and adjusted by sample dilution factor. Sample suitability testing is recommended for other biologic matrices to assess matrix interference.
Technical Specifications
| Parameter | Details |
|---|---|
| Assay format | Indirect-competitive ELISA, 96-well plate (8×12 strips) |
| Detection limit (LOD) | < 0.5 ng/mL |
| Detection range | 1 ng/mL - 16 ng/mL |
| Accuracy (Spike recovery) | 80% - 120% |
| Precision | Coefficient of variation (CV) < 20% |
| Cross-reactivity | Kanamycin 100%; Streptomycin <1%; Ampicillin <1%; Chloramphenicol <1%; Tetracycline <1% |
| Standard curve & modeling | Standard curve built from ST1-ST7; concentrations: 0.5–32 ng/mL (serial dilutions); 4-parameter logistic regression (4PL) |
| Recommended incubation temperature | 25 °C ± 3 °C (optimum) |
| Kit stability | Store kit components at 2 - 8 °C; overall kit stable for up to 12 months. Working solutions prepared fresh and used same day. |
Features
- High sensitivity: Detection limit below 0.5 ng/mL, suitable for trace-level kanamycin residue analysis.
- Good accuracy and precision: Spike recovery 80–120% and CV <20% across tested runs.
- High specificity: Minimal cross-reactivity with other common antibiotics (most <1%).
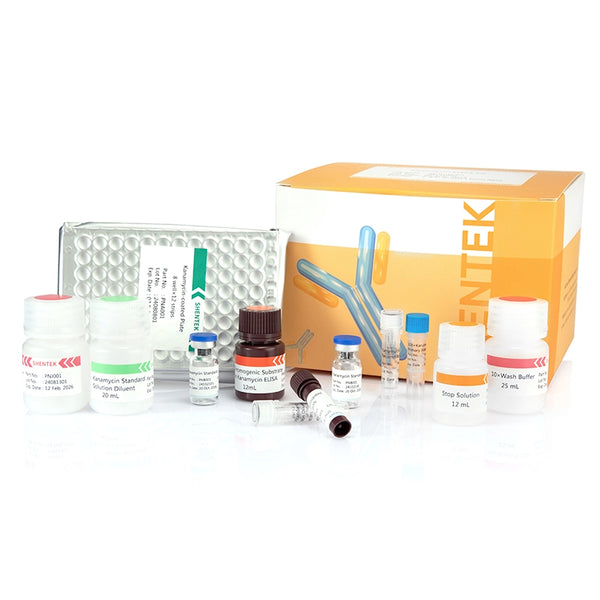
- Complete 96-test kit: Includes kanamycin-coated plate, standards, primary and enzyme-conjugated secondary antibodies, substrate and buffer reagents for 96 determinations.
- Validated workflow: Standardized procedures with 4-parameter logistic quantitation and recommended plate handling to support reproducible results.
- Optimized for biologics: Specifically intended for kanamycin quantitation in plasmid DNA and cell/gene therapy product workflows; sample suitability testing recommended for other matrices.
Applications
- Quantitation of kanamycin residues in plasmid DNA
- Quality control of cell and gene therapy products for residual kanamycin
- Research studies requiring measurement of kanamycin contamination in biologic preparations
- Screening of other biologic matrices for kanamycin after sample suitability testing
Kit Contents
Attention
• For Research Use Only (RUO); not for clinical diagnostic use.
• Store kit components at 2 - 8 °C. Do not freeze any kit components.
• Protect kanamycin standard, secondary antibody and chromogenic substrate from light.
• Prepare kanamycin standard solutions and enzyme-conjugated secondary antibody working solutions on the day of use; do not store working solutions for reuse.
• Allow kit to equilibrate at room temperature (≈25 °C) for 30 minutes before use; mix reagents thoroughly and avoid foaming.
• Maintain incubation temperature at 25 ± 3 °C; temperatures below 20 °C reduce absorbance and sensitivity.
• Do not allow wells to dry between steps; proper washing is critical—wash 4 times with 350 μL 1× wash buffer per well.
• Return unused strips to original foil bag with desiccant and seal tightly; strips stored at 2 - 8 °C up to 30 days after opening.
• Stop solution is caustic—wear eye protection and avoid contact with skin or eyes.
• Do not use kit past expiration date and do not mix reagents from different lots.
Quality Management & Certifications
Quality System
Download QMSQMS (ISO, GMP)
Download CertificateQuality Advantages
View ReportQuality Control Process
Download ProcessTechnical Resources & Downloads
Frequently Asked Questions (FAQ)
Research Use Only
Research Use Only (RUO) — not intended for clinical use – This product is intended for laboratory research purposes only and not for clinical or regulatory diagnostic use.
Not intended for use in USDA or FDA regulated diagnostic testing or official compliance testing.
When can I expect my order to ship?
Most orders are filled and shipped within 2-3 business days from the time they are received.
Our standard shipping usually take 2-5 days.
We also provide express shippping for time-sensitive deliveries.
Email contact@biofargo.com if you have any requirements.