You have no items in your shopping cart.
Description
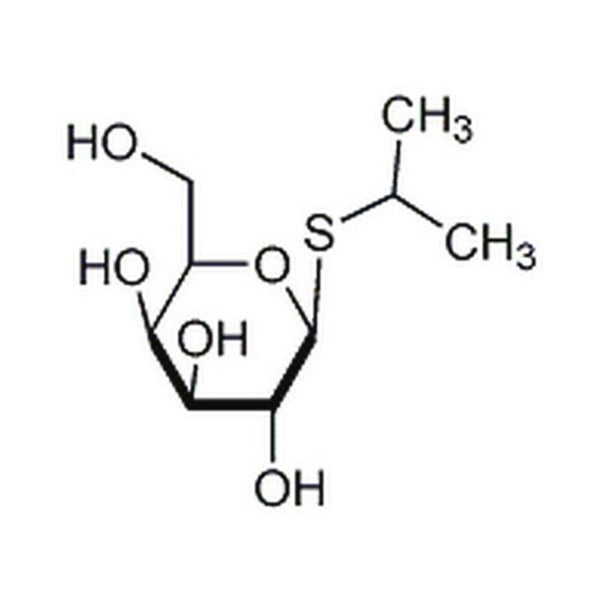
IPTG is an analog of galactose that is non-metabolizable and inactivates the lac repressor to induce synthesis of β-galactosidase in E. coli. The expression of cloned genes under the control of the lac operon is induced by IPTG. It is also a substrate for thigalactoside transacetylase and has been reported to induce penicillinase in bacteria.
IPTG is commonly used in cloning procedures that require induction of β-galactosidase and is most often used with X-Gal or Bluo-Gal for blue/white colony screening or Magenta-Gal for red/white colony screening of bacterial colonies.
IPTG is also used in the induction of recombinant proteins. In those systems, a protein of interest is encoded downstream of the IPTG inducible promoter. In the presence of IPTG, the protein of interest is induced in the cell culture. The culture can then be lysed and the protein expressed and purified through a number of methods, including His Tag or GST purification systems (for proteins with ligand tags).
Specifications
| CAS | 367-93-1 |
| Formula | C9H18O5S1 |
| MW | 238.30 g/mol |
| Grade | MOLECULAR BIOLOGY GRADE |
| Storage/handling | Store desiccated at -20°C. Protect from light. |
| PubChem Chemical ID | 656894 |
Feature
- IPTG acts by mimicking the natural inducer allolactose: it binds to the lac repressor (LacI) and causes its release from the lac operator site, allowing transcription of downstream genes under the lac promoter.
- Because IPTG is not metabolized by the cell, its concentration remains stable over time, enabling sustained induction rather than being depleted.
- In recombinant protein expression systems (for example vectors using T7 promoter under lac operon regulation), addition of IPTG triggers expression of the gene of interest downstream of the inducible promoter.
- IPTG is often used in colony screening (e.g., blue/white screening) in combination with chromogenic substrates such as X‑Gal: in this context IPTG activates β-galactosidase expression which cleaves X-Gal to yield a colored product.
- Because of the consistency and high quality of Biofargo’s IPTG, it supports reproducible gene induction workflows and reduces variability associated with induction reagent quality.
Applications
- Gene expression induction in systems controlled by the lac operon
- Blue-white screening (with X-Gal) for recombinant DNA identification
- Controlled production of recombinant proteins
- Timed gene activation for functional studies
- Regulatory input in synthetic biology circuits
Reconstitution
100mM IPTG stock solution
- Weigh 0.238 g of IPTG
- Add 10 mL sterile H2O. Dissolve completely.
- Prewet a 0.22 µm syringe filter by drawing through 5-10 mL of sterile H2O and discard water.
- Sterilize IPTG Stock through the prepared 0.22 µm syringe filter.
- Store in 1 mL aliquots at -20°C for up to 1 year.
Storage
Store powder IPTG desiccated at -20°C. Protect from light.
For IPTG stock solution, store in 1 mL aliquots at -20°C for up to 1 year. IPTG is readily dissolved in sterile water.
Documents
FAQ
Q: How does IPTG work?
IPTG, which is similar to the lactose metabolite allolactose, induces the lac operon by binding to the lac repressor. Once IPTG binds to the lac repressor, a conformational change occurs, causing the lac repressor to dissociate from the lac promoter. Once the lac repressor dissociates, RNA polymerase binds to the promoter to initiate expression.
Q: How much IPTG should be used for expression/ What concentration of IPTG should be used for expression?
Most papers recommend using between 0.1-1.0mM IPTG for expression. However, the amount you should use depends on your proteins. However, it is best to run an IPTG titration with ranges between 0.01-1.0mM and compare using SDS-PAGE to determine the optimal concentration. Something to keep in mind is higher concentrations of IPTG can cause inclusion bodies.
Q: Why should I use IPTG over lactose?
IPTG is non-metabolizable, so its concentration remains stable and doesn’t get consumed by the bacteria.
Q: When should I add IPTG during culture growth?
You should usually add IPTG at the mid-log phase (OD600 ≈ 0.4–0.6) to ensure cells are healthy and dividing rapidly.
Q: Do I need to autoclave IPTG?
No—filter sterilize only. IPTG is heat-sensitive and should not be autoclaved.
Q: Does IPTG degrade over time?
IPTG is stable when stored properly (aliquoted at -20°C, protected from light), but repeated freeze-thaw cycles should be avoided.
Q: Can IPTG be reused after thawing?
The best practice is to use single-use aliquots of IPTG. Do not refreeze IPTG leftovers to avoid degradation or contamination.
Q: Can IPTG be toxic to cells?
IPTG can be toxic at high concentrations or in sensitive strains. Lowering the IPTG concentration or using auto-induction media can help.
Disclaimer
For laboratory research use only.
When can I expect my order to ship?
Most orders are filled and shipped within 2-3 business days from the time they are received.
Our standard shipping usually take 2-5 days.
We also provide express shippping for time-sensitive deliveries.
Email contact@biofargo.com if you have any requirements.