You have no items in your shopping cart.
Product Description:
Animal Tissue Cell Total RNA Extraction Kit employs a rapid, phenol-free and chloroform-free RNA extraction technique for swift extraction of total RNA from easily lysable animal tissue and cell. The lysis buffer in this kit rapidly lyses cell and inactivates RNase, without the need for β-mercaptoethanol from conventional kits. The unique genomic DNA removal column guarantees effective removal of gDNA residue, eliminating the need for DNase digestion and allowing direct use of RNA for tests such as RT-PCR and qPCR. If downstream experiments are highly sensitive to trace DNA contamination, additional DNase treatment can be employed.
This kit extracts high-purity RNA fast and easily. Generally, the operation of a single sample can be completed within 30 minutes.
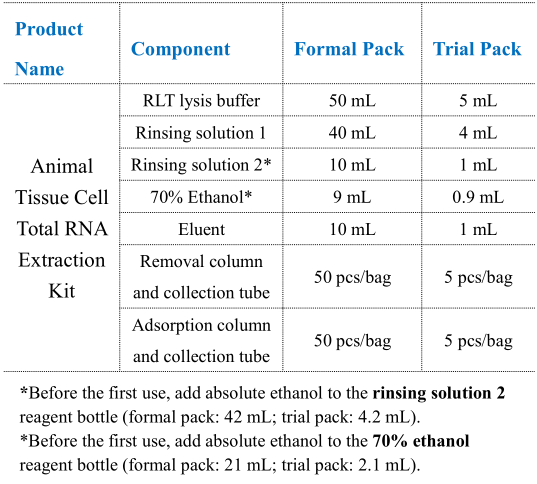
Storage and Transportation:
Store at 2°C - 30°C, with a shelf life of 12 months.
Transport at room temperature.
Scope ofApplication:
The product can only be used for in vitro research
and not for diagnostic purposes.
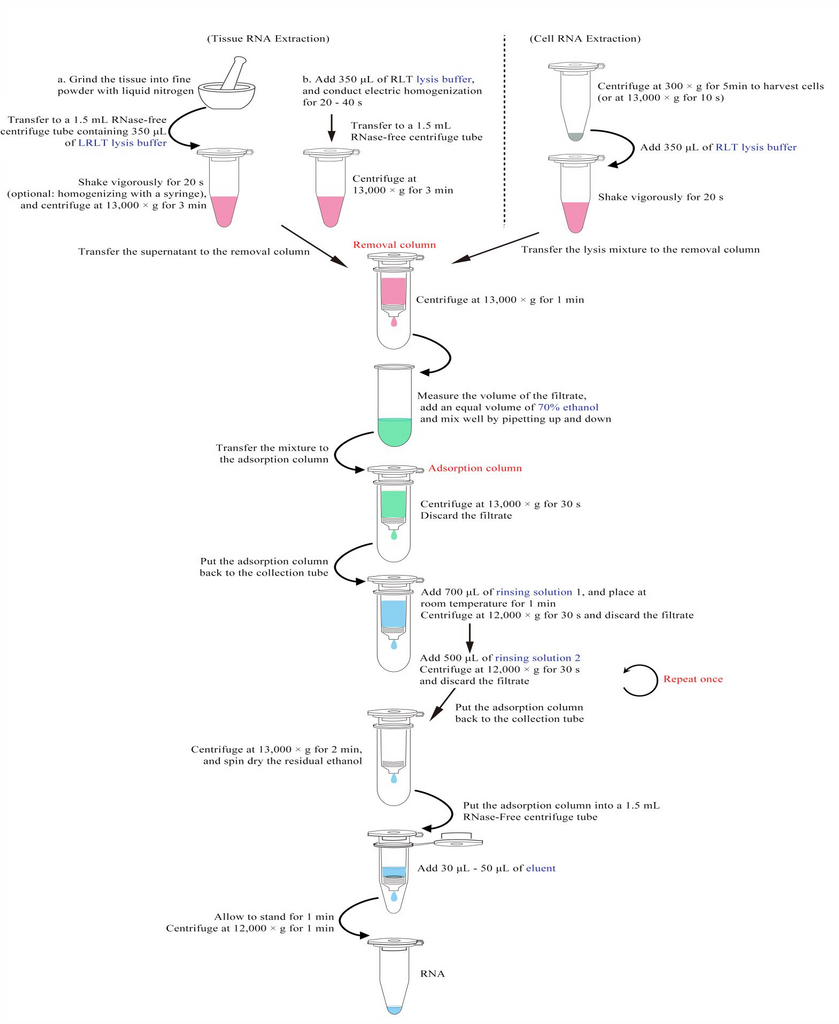
Extraction Steps of Cultured Animal Cell Total RNA: (please read the precautions before the test).
1. Collect ≤ 1 × 10 7 cells by centrifuging at 300 × g for 5 min or 13,000 × g for 10 s, and remove supernatant.
2. Add 350 μL (< 5 × 10 6 cells) or 600 μL (5 × 10 6 - 1 × 10 7 cells) of RLT lysis buffer, mix well by pipetting up and down, shake vigorously by hand for 20 s, and add all the lysis mixture to the removal column, which is put into the collection tube.
3. Centrifuge at 13,000 × g for 1 min and retain the filtrate.
Note: Ensure that all liquid is filtered after centrifugation. Increase the centrifugal force and time if necessary.
4. Accurately measure the volume of the filtrate with a pipette, add an equal volume of 70% ethanol and mix well by pipetting up and down.
Note: Check whether absolute ethanol has been added to the reagent bottle.
5. Immediately transfer the mixture to the adsorption column, which is put into the collection tube, centrifuge at 13,000 × g for 30 s, and discard the filtrate.
Note: Do not transfer more than 700 μL per time. If more than 700 μL of mixture needs to be transferred, do it in two separate transfers and centrifuge each time.
6. Add 700 μL of rinsing solution 1, place at room temperature for 1 min, centrifuge at 12,000 × g for 30 s, and discard the filtrate.
7. Add 500 μL of rinsing solution 2, centrifuge at 12,000 × g for 30 s, and discard the filtrate.
Note: Check whether absolute ethanol has been added to the reagent bottle.
8. Repeat Step 7.
9. Put the adsorption column back into the collection tube and centrifuge at 13,000 × g for 2 minutes to completely remove residual ethanol via spin-drying.
Note: The presence of residual ethanol can inhibit downstream reactions.
10. Put the adsorption column into an RNase-free 1.5 mL centrifuge tube, add 30 μL - 50 μL of eluent to the center of the adsorption column membrane, allow it to stand for 1 min, and centrifuge at 12,000 × g for 1min.
Note: The yield can be improved by preheating the eluent in a water bath at 50°C - 70°C. If the expected RNA yield exceeds 30 μg, suck the eluent back into the adsorption column and repeat Step 10; or add another 30 μL - 50 μL of eluent and repeat Step 10, and mix the two eluents.
Extraction Steps of Animal Tissue Total RNA: (please read the precautions before the test)
1. Samples are first electrically homogenized or ground with liquid nitrogen.
a. Electric homogenization: Quickly cut fresh tissues into small pieces with a scalpel, add 350 μL (< 20 mg of tissue) or 600 μL (20 mg - 30 mg of tissue) of RLT lysis buffer, and then electrically homogenize the mixture thoroughly for 20 - 40 s.
b. Grinding with liquid nitrogen: Grind the tissue into fine powder with liquid nitrogen, transfer an appropriate amount of fine tissue powder (20 mg/30 mg) into an RNase-free 1.5 mL centrifuge tube containing 350 μL/600 μL of RLT lysis buffer, and shake the tube vigorously by hand for 20 s to fully lyse.
Optional steps: Use a disposable 1 mL (with a 0.9 mm needle) syringe with a blunt needle to vigorously push and pull the plunger at least 10 times until a satisfactory homogenization effect is obtained, so as to shear DNAand reduce viscosity to prevent column plugging and improve yield.
2. Centrifuge the homogenized mixture at 13,000 × g for 3 min, and transfer all the supernatant to the removal column, which is put into the collection tube.
3. Follow Step 3 of extraction of cultured animal cell total RNA.
Operation Steps:
Precautions:
1. Please read the operating instructions carefully before use.
2. Before the first use, add absolute ethanol to the rinsing solution 2 reagent bottle (formal pack: 42 mL; trial pack: 4.2 mL); add absolute ethanol to the 70% ethanol bottle (formal pack: 21 mL; trial pack: 2.1 mL).
3. Ensure that all solutions are clear before use. Low-temperature storage can cause precipitation and affect the use effect. The solution can be restored to clarity in a water bath at 37°C for several minutes.
4. Perform all centrifugation at room temperature.
5. The sample processing volume should not exceed the processing capacity of the genomic DNA removal column and RNA adsorption column to avoid DNA residue or yield reduction. The RNA/DNA of various samples differs greatly, leading to a variation in the sample processing volume. When the experiment conditions are preliminarily explored, limit cell samples to 3 - 4 × 10 6 and tissue samples to 10 mg. Determine the processing volume for subsequent experiments based on the results.
6. If residues are caused by excessive DNA content or rigorous fluorescent quantitative PCR is required, it is recommended to select intron-spanning primers or treat the RNAextract with RNase-free DNase.
7. For the samples with excessively high DNAcontent, Step 6 can be performed as follows:
-
a) Add 350 μL of rinsing solution 1 to the adsorption column, centrifuge at 12,000 × g for 30 s, and discard the filtrate (putting the adsorption column into the collection tube).
-
b) Add an appropriate amount of DNase working solution to the center of the adsorption column and place it at room temperature for 15 min.
-
c) Add 350 μL of rinsing solution 1 to the adsorption column, centrifuge at 12,000 × g for 1 min, and discard the filtrate (putting the adsorption column into the collection tube).
8. Ensure that equipment used in the experiment is RNase-free, including gloves, pipette tips, EP tubes and mortars.
9. Bake the glassware at 150°C for 4 hours. Soak the plasticware in 0.5 M NaOH for 10 min, wash it thoroughly with water, and sterilize it to remove RNase.
10. The product is only intended for scientific research conducted by professionals. For your safety and health, please wear lab coats and disposable gloves during the operation.
Document
When can I expect my order to ship?
Most orders are filled and shipped within 2-3 business days from the time they are received.
Our standard shipping usually take 2-5 days.
We also provide express shippping for time-sensitive deliveries.
Email contact@biofargo.com if you have any requirements.



