You have no items in your shopping cart.
Product Description
Mouse Lymphocyte Separation Medium is a new generation of density gradient separation medium. The main component is iodixanol, with a molecular weight of 1,550. It is a completely chemically inert, non-biotoxic iodide that does not bind with any known biological function protein, does not interfere with any cell surface membrane protein, does not inhibit enzyme activity and does not interfere with antigen-antibody responses.
The lymphocyte separated by this product is with high purity, good state and high yield. The operation of Mouse Lymphocyte Separation Medium is simple, easy to learn and does not need too much experience.
Our study showed that the number and quality of mouse’s spleen lymphocytes did not change significantly after 1 hour of exposure to the separation medium, and the subsequent ELISPOT test results were completely consistent with those of the control group.
Product Information
| Product name |
Mouse Lymphocyte Separation Medium | Size | 7211011:100mL/bottle 7211012:250mL/bottle |
| Application | Separation of mouse/rat spleen lymphocytes Separation of mouse/rat/rabbit PBMCs |
Endotoxin | < 0.5EU/mL |
| Density | (1.081±0.001) g/mL (20 °C) | Osmolality | (280±15)mOsmol/kg |
Storage:The product shall be stored at 2 °C -30 °C and protected from light. Its shelf life is 2 years.
Materials Required But Not Provided
35mm Petri dishes, the plunger of a 10mL glass syringe, 70µm cell strainer or nylon mesh with 200 meshes - cut into 90mm×90mm square (materials above are all sterile), centrifuge tubes, pipettes, tips, centrifuge with a swing-out rotor, etc.
Protocol
1. Sacrifice the mouse by cervical dislocation; dip it in 75% ethanol.
2. Take out the spleen of the mouse at a clean bench. Operation shall be performed in an aseptic condition.
3. Add 4-5 mL Mouse Lymphocyte Separation Medium (pre-warming at room temperature and shaking before use) into a 35 mm petri dish. And grind (please refer to Figure 2 for grinding operation).
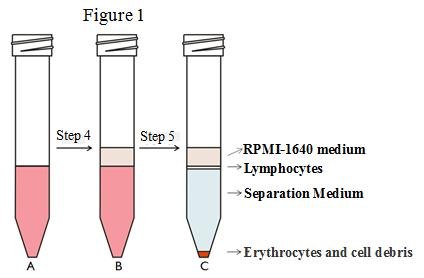
4. Transfer the cell suspension to a 15mL centrifuge tube immediately (refer to
Figure 1(A)), and cover with 500-1000μl RPMI 1640 medium (keeping liquid level boundary clear). Please refer to Figure 1(B).
5. Centrifuge at 800g for 30min in a swing-out rotor at room temperature. The acceleration and deceleration are set to a slower speed (set to third gear if ten gears are available). The lymphocytes after centrifugation are shown in Figure 1(C).
6. Transfer the lymphocyte layer at the interface to a new centrifuge tube. Wash the cells with 10mL RPMI 1640 medium, and centrifuge at 250g for 10min.
7. Discard the supernatant, resuspend the cells in culture medium and count.
Cautions
● If the mouse is fed for a long time or the spleen is enlarged abnormally, which causes that the cell suspension after grinding shows dull red, please dilute the cells suspension with an equal volume of Mouse Lymphocyte Separation Medium and mix it well, and then proceed to step 4.
● The separation medium is volatile so the grinding time shall be controlled within 5 min.
● Once opened, please store the separation medium at 2-8℃ to avoid the change of density of the separation solution caused by liquid volatilization, which will affect the separation effect.
Separation of the spleen lymphocyte of the rat:
Because the rat’s spleen is large, only a small portion of it needs to be cut for the experiment. The method of grinding and separation is exactly the same as that for the separation of mouse’s spleen lymphocytes.
Separation of mouse/rat/rabbit blood lymphocyte:
1. Collect 0.5-3mL anticoagulant blood of mouse/rat/rabbit, dilute it with
an equal volume of RPMI 1640 medium or PBS. (Separation is more
effective after hemodilution. Operation shall be performed in an aseptic
condition)
2. Add 3mL of Mouse Lymphocyte Separation Medium to a 15 mL centrifuge tube. Carefully layer the diluted blood over 3mL Mouse Lymphocyte Separation
Medium in a 15mL centrifuge tube, avoid mixing the interface.
3. Centrifuge at 800g for 15-20min in a swing-out rotor at room temperature. The acceleration and deceleration are set to a slower speed (set to third gear if ten gears are available).
4. The subsequent steps are the same as the mouse’s spleen lymphocyte separation operation.
Special instruction - Mouse’s spleen grinding:
● It is recommended to use nylon mesh, because it is more flexible.
● It is recommended to use the plunger of syringe to grind the spleen.
● The key is taking advantage of the rebound to control grinding, which can minimize the mechanical damage of the cells.
Notes
1. Control the grinding strength to keep the nylon mesh suspended, to avoid a large number of cell death caused by direct grinding on the bottom of the petri dish.
2. The petri dish cannot be too large, otherwise the nylon mesh cannot have effective elasticity.
3. Clamp the nylon mesh with tweezers on one side to prevent the nylon net from sliding during grinding.
References
1. Lili Liu, et al.American Journal of Nephrology. 2012; 36(2): 386-396
2. Li Zhang, et al. Iranian Journal of Allergy, Asthma and Immunology. 2012; 11(2): 133-145
3. JianHui Xiao,et al.Evidence-Based Complementary and Alternative Medicine.2012;ArticleID 273435, 15 pages
Document
When can I expect my order to ship?
Most orders are filled and shipped within 2-3 business days from the time they are received.
Our standard shipping usually take 2-5 days.
We also provide express shippping for time-sensitive deliveries.
Email contact@biofargo.com if you have any requirements.


















